Equipment & Protocol
Standard Referenced Oximeters
> Nellcor N-560
> Masimo Radical-7

Blood Gas Analyzer
> Radiometer ABL90

Computer Gas Control System
Labview Computer monitoring system: Record all sampling and reading changes of saturation values of the tested oximeter during the test process by connecting the computer control system and the standard referenced oximeter.
A standard arterial catheter will be introduced into the radial artery of the wrist or foot (left or right) by the Investigator after local anesthesia for subsequent blood draws. The volunteer will be coached to lie down on the test bed and will be covered with a blanket to maintain constant body temperature. Test oximeter sensors shall then be placed on their fingers. Finger covers can be placed over the sensors to avoid external interference. One finger will be reserved for the sensor of the referenced oximeter and its reading should achieve a stable SpO2 value. The gas controller gets the control system ready, affixes a nose clip on either side of the nostrils, so that he/she is forced to breathe through a mouthpiece. With the gas controller’s coaching, mixed gases containing air and CO2 is introduced into the the volunteer’s airway. The gas controller is then able to control the flow rate through the monitoring system accordingly to reduce oxygen content to achieve stabilized plateaus at various lower saturation levels. The following are value targets: The target samples to obtain at each plateau will be 2 samples. The objective of these targets is to spread the data points evenly over the desired range. Achieving them exactly is not important, though every effort will be made to be within 2%.
Run 1 Current SpO2 value (under room air) 92%, 87%, 82%, 77%, 72%
Run 2 100% (under pure oxygen), 93%, 88%, 83%, 78%, 73%
During the test, Nellcor N560, Masimo Radical V4 or other brands with the same function and sensors are usually used to obtain the reference value of current saturation.
With the decrease of FiO2 (inhaled oxygen concentration), the oxygen saturation value and end tidal oxygen partial pressure curve of the reference oximeter begin to change and reach a stable plateau. Samples can be taken after achieving the plateau and stabilizing for 45s (the reading of the reference oximeter does not change by more than 1% in 45s). Draw about 1ml blood sample from the indwelling tube and record the value of the equipment; After 30s, a 1ml blood sample is taken and the reading of the oximeter is recorded. Repeat the above test steps at every desired stabilized plateau. Maintain the stable plateau for at least 45s in order to achieve a stable SaO2 saturation in between plateaus.
Throughout the test, about 20-25 blood samples is taken (approx. 1ml each) from the arterial catheter placed on the wrist of the volunteer. The total test duration carried out below room air saturation should not exceed 45 mins. The data from 12 volunteers will produce 288 blood samples which surpasses standard requirements of 10 volunteers and 200 data sets by US FDA, CE and NMPA.
Data collection during clinical tests are done manually due to a tight test time frame. In order to avoid mistakes, there should be one person calling out values and one person recording. The data shall at least include SpO2 data at each blood draw, data of monitoring equipment (SpO2 value), SaO2 value of blood gas analyzer, etc.
Data analysis is done according to standard recommended methods, taking the blood gas measurement results as the referenced value for paired statistical analysis. The number of paired groups in the statistical calculation should be no less than 200 groups. Calculate the relevant parameters according to a 10% interval in SpO2 values and multiple measurement ranges, such as 60 ~ 70%, 70 ~ 80%, 80 ~ 90%, 90 ~ 100%, 60 ~ 100%, 70 ~ 100% and 80 ~ 100%. Using Excel statistical method, the standard deviation is calculated for all effective data, taking the blood gas analysis value as the referenced value and the monitoring value of the tested oximeter as the value to be verified. The calculation formula is as follows:
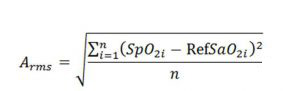
SpO2i is the reading value of the tested oximeter for the nth time, and SaO2i is the corresponding synchronous blood gas analysis value for the nth time. In addition, missing, unused or erroneous data and unreasonable data elimination have to be explained and not participate in statistical calculation. Retention of such records is needed in the table of data recording.
Finally, the lab will issue a clinical test data report according to required format and content




