Volunteer Recruitment Notice
SUGH is fully invested and built by Shenzhen Municipal People’s Government. It is an internationally renowned and domestic first-class research hospital integrating medical treatment, teaching, scientific research and health management. It is the First Affiliated Hospital of Shenzhen University. This study follows the requirements of Announcement No. 25 of the State Food and Drug Administration on the Quality Management of Clinical Trials of Medical Devices issued in 2016, the special requirements for the Basic Safety & Basic Performance of Pulse Oximeter Qquipment in ISO 80601-2-61:2011, and the moral principles of Helsinki Declaration of the World Medical Association. Pulse oxygen saturation is one of the important physiological parameters of human body. Pulse oxygen saturation monitoring has seen clinical applications worldwide for more than 30 years. This study aims to verify the accuracy of pulse oxygen saturation and the safety and effectiveness of the tested devices.
According to article 50.101 of YY 0784-2010 Special Requirements for Basic Safety & Performance of Medical Electrical Equipment & Medical Pulse Oximeters (implemented on June 1, 2012) and the Requirements of Technical Guidelines for Clinical Evaluation of Pulse Oximeters issued by the State Food and Drug Administration on February 18, 2016 and ISO 80601-2-61:2011 Special Requirements for the Basic Safety & Performance of Pulse Oximeters and to carry out clinical verification of the accuracy of pulse oxygen saturation, safety and effectiveness of the product at the same time.
The function and performance of pulse oxygen saturation measurement equipment are verified using standard requirements of test comparison method, and the performance and safety of the test equipment are determined by statistical methods and the results obtained.
For enquiries, please contact our lab research coordinator.
Address: Block A5, Unit 204, So Fun Land, Taoyuan Community, Nanshan District, Shenzhen.
Enquiry Hotline
- Phone: 0755-86170552
- Ms. Huang: 13556869952
- Mr. Cai : 13528442155
- Adults aged 18-50; Males & females included;
- Non-smokers & history of smoking;
- No previous history of cardiopulmonary disease;
- Ability of independent behavior, voluntary participation in current study & sign Informed Consent Form;
- Blood Pressure range: Systolic blood pressure 90 ~ 140mmHg, Diastolic blood pressure 60 ~ 90mmHg;
- Heart rate: 60-100bpm;
- SaO2 of volunteer in room air: SaO2>95%, COHb<3%, MetHb<2%, CTHb>10g/dl;
- Routine physical examination screening passed (screening items include height, weight, internal medicine, surgery, urine test, ECG, chest X-ray and blood test (hemoglobin)).
Test Procedure :
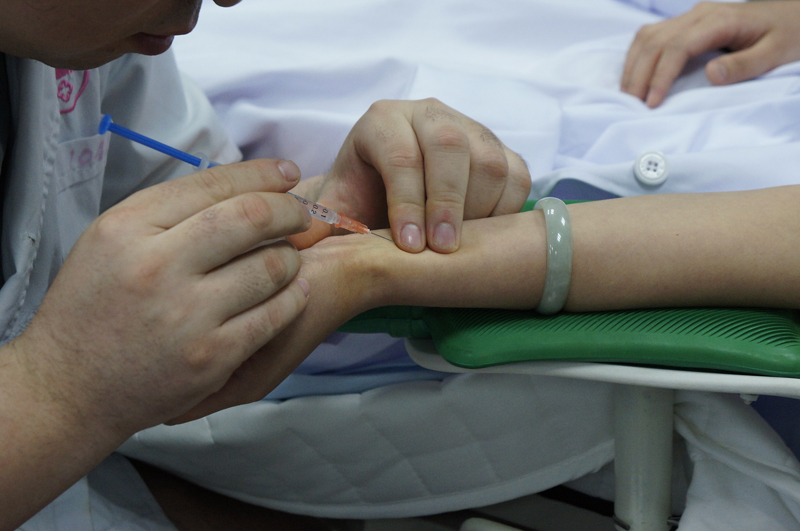
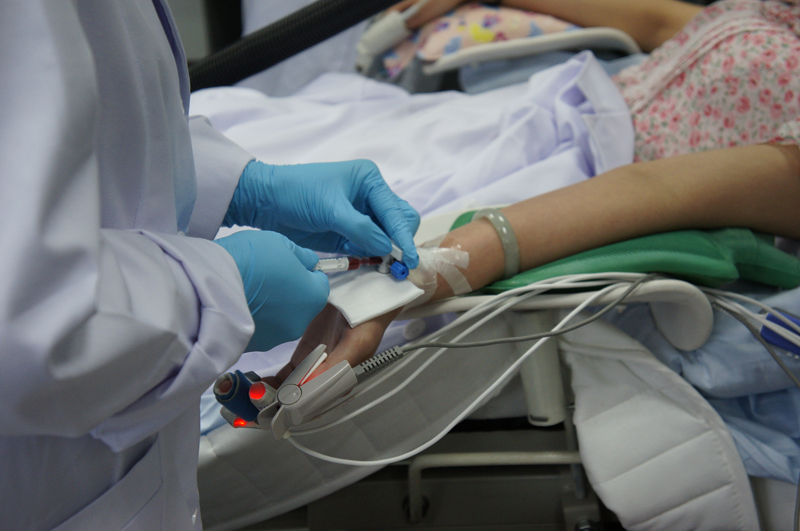
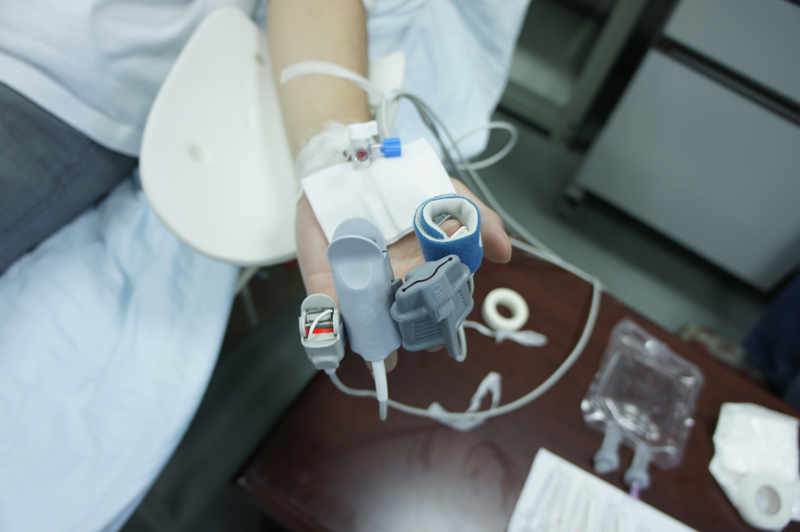
You (volunteer) shall be asked to lie down or sit comfortably on the test bed. Sensors of the tested devices and referenced oximeter will be positioned on your fingers, forehead, ear lobes or other placement sites on your body. You will have your nose clipped tight and will breathe through a mouthpiece connected to a breathing circuit. The circuit is connected to a multiple-gases control system where mixed gases will be controlled and lowered to several hypoxic levels (12-21%). This will gradually lower your blood oxygen saturation from 100% to 70%. This controlled state of gradual oxygen reduction consists of different cycles and several saturation plateaus. At the same time, approx. 1ml of blood is drawn from the arterial catheter positioned on your radial artery (3 blood draws at every stable plateau, the first being an unwanted sample while the second and third, 30s apart, being effective samples). The total blood draw volume is approx. 36ml upon completion of 24 effective blood samples and the total test duration for each volunteeris approx. 45 mins (inclusive of blood sampling and recovery time).
The lab compensates volunteers participating in the study with work delay, meal and transportation subsidies.
- Test Location : Block A5, Unit 204, So Fun Land, Taoyuan Community, Nanshan District, Shenzhen.
- Phone : 0755-86170552; Ms. Huang : 13556869952; Mr. Cai : 13528442155
Our test protocol requires participation of volunteers with various skin types/colour.
Volunteers with skin colour Types 4, 5 and 6 on the Fitzpatrick Scale are specially invited.